-
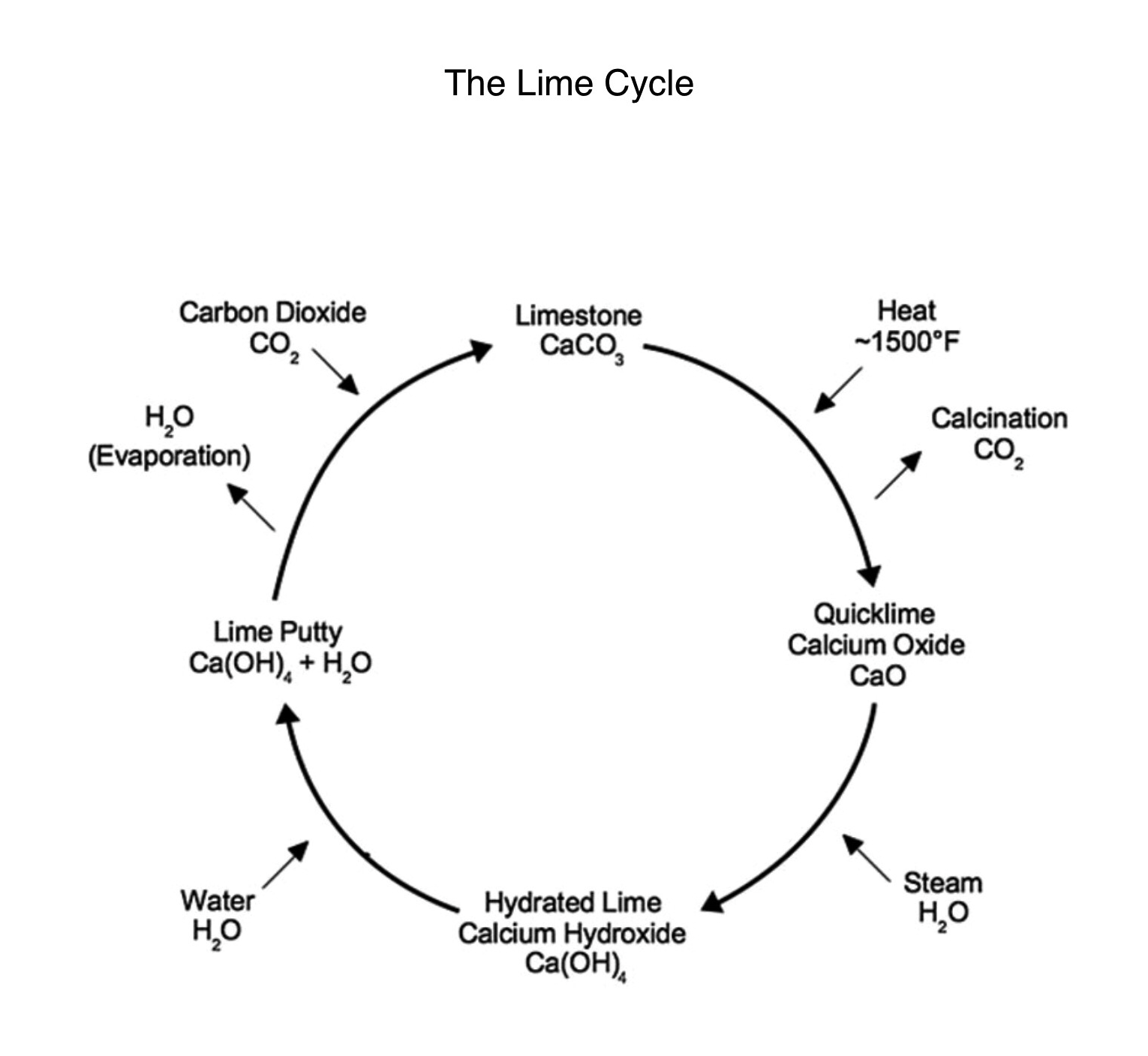
The Lime Cycle
Lime has been used for thousands of years as the binder in mortars in all kinds of construction, and when it comes to lime mortar it’s all about the lime cycle.
Lime starts off as limestone (calcium carbonate – CaCO3) then heat is added to around 900c to convert limestone into quicklime (calcium oxide – CaO) at the early stage the water is vaporised into a gas and as it heats up the C02 is driven out to become quicklime.
Next water is introduced (slaking) and this is when it creates its own heat (exothermic reaction) and turns to lime putty. In time the lime putty (calcium hydroxide – Ca(OH)2) turns into a lovely creamy texture, with age it matures and the better it becomes.
Now we introduce the aggregate by knocking up to a workable consistency, once worked it’s all about the curing (carbonation) this is another very important part of the procedure and also often overlooked.
Once cured is when it has converted back to limestone from which it originated, The Lime Cycle
-